Traditional treatments for repairing a damaged or torn spinal disc leave patients with a host of side effects, and don’t consistently provide relief. Discseel® is a revolutionary procedure using an FDA approved biologic to repair your damaged spinal disc.
It is a minimally invasive procedure that injects an FDA approved biologic into intervertebral discs. This is able to repair and seal damaged spinal discs, where spine surgery, including spinal fusions, can’t.
Your intervertebral discs are oval structures that work as cushions between your spine bones (vertebrae). These discs are made of two regions: the inner jelly-like region (Nucleaus pulposes) and the outer harder rim (Annulus Fibrosis).
Due to age, disease or trauma, the outer rim of intervertebral discs undergoes wear and tear. The outer rim, (Annulus Fibrosus) can tear leading to leaking of the soft center out into the intervertebral canal. This frequently leads to problems like nerve irritation and back pain. Sometimes the tears are microscopic while other times they can be obvious.

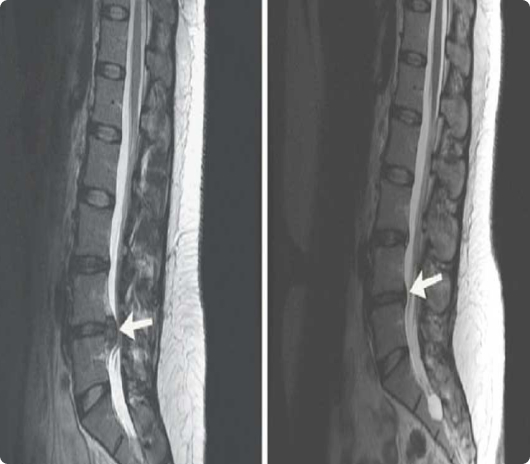

A 2020 study of 320 patients that is pending publication reports that: “Statistically significant improvement was realized in every outcome tool comparing baseline to; 3-months, 6-months, and 1-year post-treatment. No subject was lost to follow-up or withdrawn, and no subject experienced an adverse event. The results of the longitudinal analysis demonstrated statistically significant improvement in all outcomes measured.”
Any person with long lasting low back pain is a potential candidate. A person with low back pain who’s had surgery or a person with low back pain who prefers to avoid surgery is also a potential candidate, because the cushion in the back known as the disc is the most common cause of low back pain. Contact us to find out if you are a candidate.
No. We use conscious sedation during the procedure, so it is not painful or uncomfortable for our patients. Conscious sedation is a combination of medicines to help you relax (a sedative) and to block pain (an anesthetic) during a medical procedure. Under conscious sedation, you may fall asleep, but you will wake up easily to respond to people in the room. You may be able to respond to verbal instructions. After conscious sedation, you may feel drowsy and not remember much about your procedure, but you will recover quickly and we encourage you to begin walking the next day.
This is an outpatient procedure done in a morning or afternoon. You will likely need a few days to rest, however, we want you up and walking around the day following your procedure. After the first few days, when you are feeling up to it, we want you to begin a daily regimen of walking, gradually building up your walking distance every few weeks. Most people need prescription pain medication for the first few weeks.
Most people see a change within 3 – 6 months after the Discseel® procedure, and some are sooner than that. Most people are back at work the week after their procedure. It is normal to experience increased symptoms after your procedure and then go through a period of time where the pain waxes and wanes for several months.
The main thing to remember following your procedure is to avoid two movements: flexion (forward bending) and rotation (twisting). Flexion is a compressive force and rotation is a shear force, both of which can damage and tear discs. Many people ask, “How long do I have to avoid those movements?” We encourage all patients to avoid these movements as a lifestyle because we know these are the two forces that can damage and tear discs. We recommend working with a physical therapist for a short period of time following the procedure to learn new body mechanics in order to avoid movements that cause wear and tear on the discs.
It can take 3 – 12 months for the disc to be restored and for you to have noticeably decreased pain and improved function. Beginning the day of your procedure, your discs will begin the healing process. Most patients experience increased symptoms following the procedure for several weeks and do not notice significant change from their usual pain for 3-6 months. Occasionally some notice a quick difference, but that is the exception, not the norm.
We will work with your prescriptions and provide pain medications immediately following your procedure. If you are an out of state patient, because of pharmacy regulations, we ask that you follow up with a pain management doctor in your hometown before your prescription runs out if you need to continue medications to manage your pain.
Intradiscal injection of fibrin sealant for the treatment of symptomatic lumbar internal disc disruption: results of a prospective multicenter pilot study with 24-month follow-up. Yin W, Pauza K, Olan WJ, Doerzbacher JF, Thorne KJ.; Pain Med. 2014 Jan;15(1):16-31. doi: 10.1111/pme.12249. Epub 2013 Oct 23. Link to article
Discseel® Regenerative Spine Procedure Website. Link to website
Treatment of annular disc tears and “leaky disc syndrome” with fibrin sealant. Pauza K MD, Wright C BS, Fairbourn A Best, Mark.; Techniques in Regional Anesthesia and Pain Management Vol 19, Iss 1–2, Jan–Apr 2015: 45-49. Link to article
Intradiscal Biologic Treatments: Intra-annular Fibrin Disc Sealant. Kevin Pauza MD; Advanced Procedures for Pain Management: A Step by Step Atlas; Ed: Diwan S , Deer TR, 1st Ed, 2018 Edition pp 525-536.
Fibrin Injection Stimulates Early Disc Healing in the Porcine Model. Buser Z, Kuelling F, Jane L, Liebenberg E, Tang J, Thorne K, Coughlin D, Lotz J; The Spine Journal 9(10) · October 2009 Link to Article
Spinal Fusion for Chronic Low Back Pain: A ‘Magic Bullet’ or Wishful Thinking? Dhillon KS.; Malays Orthop J. 2016 Mar; 10(1): 61–68.